Histolndex Contributed to the Development of the First FDA-Approved MASH Treatment
The Road to Rezdiffra
After many years of development, Madrigal received accelerated approval from the FDA for Rezdiffra™ (resmetirom), making it the first and only medication approved for the treatment of NASH (also known as “MASH”) in patients with noncirrhotic NASH with moderate to advanced liver fibrosis. HistoIndex contributed to the Rezdiffra development program by partnering with Madrigal on exploratory analyses of Phase 2 and Phase 3 study data.
The Challenge
MASH is a challenging disease to treat because of its heterogeous nature and complexity, and several drug candidates have failed in clinical trials due to various factors, such as:
- Limitations of liver histology such as poor intra-and inter-reader reliability, and the lack of standardization in biopsy reading
- Challenges in selecting the appropriate target population and stratifying patient
- Need for determining the ideal trial duration, which is dependent on their mechanism of action, severity of the targeted population, and magnitude of effects

Our role: Digital Pathology for Fibrosis Assessment
Quantified Fibrosis
Histolndex’s proprietary technology, qFibrosis, was incorporated in Madrigal Pharmaceuticals’ Phase 2 and Phase 3 clinical trials for Rezdiffra as exploratory endpoints. Combined with label-free second harmonic generation (SHG) microscopy to visualize fibrillar collagen, qFibrosis offers a unique, quantitative approach to assess fibrosis improvement by analysing spatially, the unique morphological and architectural changes of every collagen fibre as well as the overall featural changes in the collagen network.
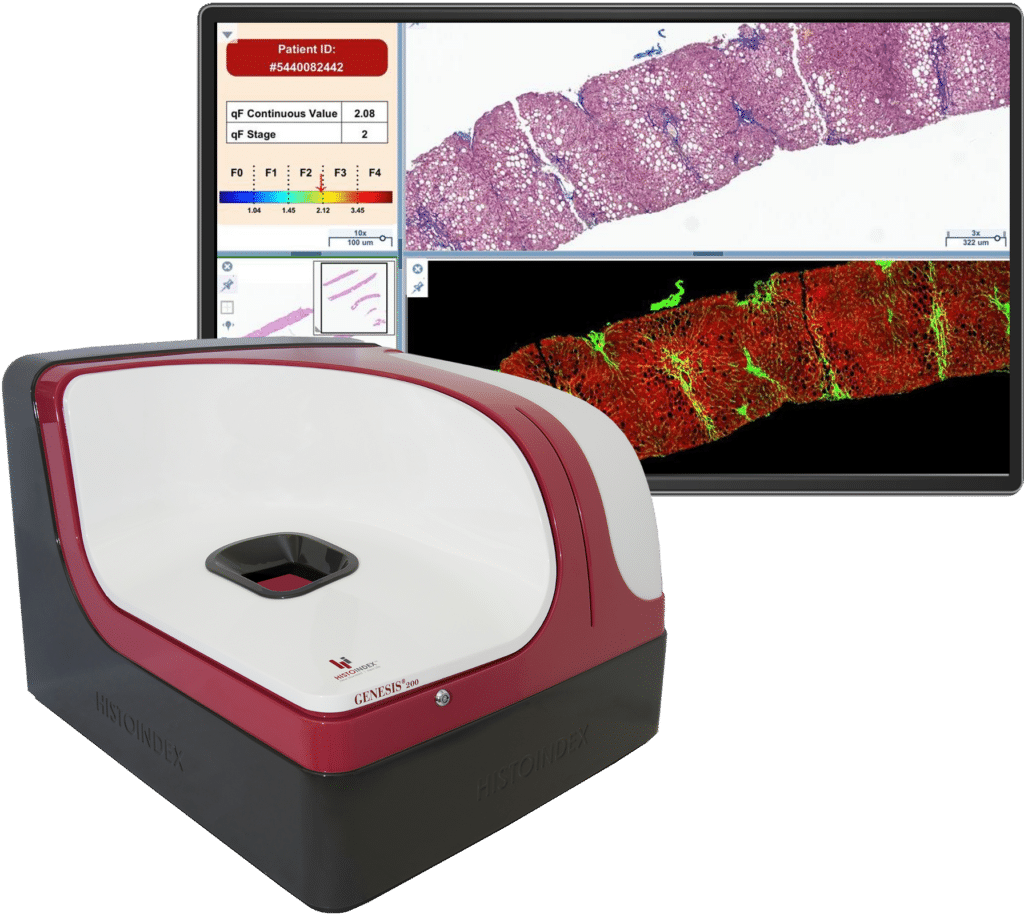
Our Insight
qFibrosis revealed that baseline F3 patients were the best responders to Rezdiffra in the Phase 2b trial. About half of F3 patients showed ≥ 1-point improvement in fibrosis, compared to placebo F3 patients.
Translating Phase 2 Insights to Phase 3 Study Design
Trailblazing Results
The Phase 2 qFibrosis results allowed for powering of the Phase 3 trial to include more than 51% of F3 patients and an adjustment of the Phase 3 trial duration to 52 weeks. In the Phase 3 trial, Rezdiffra demonstrated significant improvement in both primary endpoints: MASH resolution and ≥1 stage fibrosis improvement. AI-based measurements of fibrosis change using qFibrosis showed
- Reduction in fibrosis in both Rezdiffra 80 and 100 mg groups relative to placebo.
- A clear improvement and less worsening in fibrosis in Rezdiffra-treated patients as compared with placebo after 52 weeks of treatment.

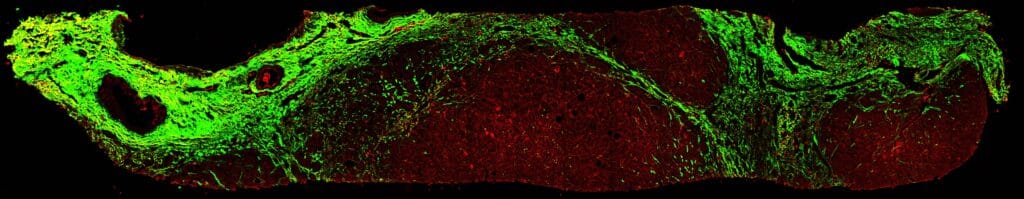
Our Impact on Drug Development
A New Era in MASH Clinical Trials
This collaboration showcased the potential for more accurate and comprehensive assessment of drug efficacy in MASH clinical trials using stain-free imaging and quantitative assessments of fibrosis. AI-based digital pathology improves clinical trials and accelerates the development of effective treatments for MASH.
