HistoIndex and CymaBay Therapeutics Team up to Advance NASH Drug Development
Histoindex Pte. Ltd., with the world’s first stain-free Genesis®200 multiphoton digital pathology system, is collaborating with CymaBay Therapeutics, Inc. to evaluate the efficacy of seladelpar in a randomized, double-blind, placebo-controlled, multi-center Phase 2b study of 175 subjects with biopsy-confirmed Nonalcoholic Steatohepatitis (NASH).
Genesis®200’s novel imaging technology encompasses Two-Photon Excitation (TPE) and Second Harmonic Generation (SHG), and works with computerised image analysis algorithms to scan unstained tissue samples from liver biopsies and quantify the NASH characteristics within. The vivid high-resolution images generated are rich in histopathological data, which pathologists will be able to extract and analyse, for example, sub-micron-sized collagen fibers, which indicate the presence and severity of fibrosis in NASH.
Other features that are quantified include steatosis, hepatocyte ballooning, and lobular inflammation. Assessing the progressive or regressive changes to these quantified features is vital in determining the NASH therapeutic response of seladelpar.
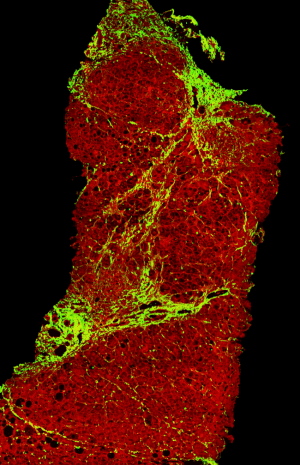
Figure A: Seen here is a scanned image of an unstained liver tissue sample, where the collagen fibers are highlighted in green, thus indicating the occurrence of fibrosis.
The current standard of assessment and evaluation is conventional staining with manual observations and histopathological scoring, and here is where limitations, such as intra- and inter-observer variability in assessment, deter the ability to detect minute changes in fibrosis, steatosis, ballooning and inflammation. Therefore, having a precise and well-validated tool that can assess and evaluate – with improved accuracy and sensitivity – the full spectrum of NASH characteristics as well as any incremental improvements to them is crucial in pharmaceutical drug development and clinical trials.
Says Dr. Gideon Ho, CEO of HistoIndex, “We are enthusiastic to work with CymaBay, and our passion synchronises with their dedicated efforts to bring effective NASH therapeutics to patients. I am confident that our imaging technology will allow us to accurately quantify and track changes in fibrosis and relevant NASH characteristics such as steatosis, hepatocyte ballooning and lobular inflammation.”
Mr. Sujal Shah, CEO of CymaBay, adds, “We are excited to be working with HistoIndex in our Phase 2b study of seladelpar in patients with NASH. We believe this collaboration offers us a unique opportunity to explore the Genesis®200 system to generate an innovative, data set evaluating the effects of seladelpar on NASH histology.”
Dietary and lifestyle changes are the only available options for patients diagnosed with NASH as drug treatments are currently not yet available in the market. NASH is expected to be the leading cause of liver transplants by 2020. Hence, the crucial need for effective treatments remains to be met.
