A New Standard in Clinical Care for MASH Patients: HistoIndex Launches FibroSIGHT™
SINGAPORE, March 17, 2025 /PRNewswire/ — HistoIndex, a global leader in stain-free digital pathology solutions for managing fibrotic diseases, has announced the launch of their first Laboratory Developed Test (LDT) FibroSIGHT™ – now available in the United States. This marks a significant milestone for HistoIndex as the company enters clinical care for patients with Metabolic Dysfunction-Associated Steatohepatitis (MASH). “I am excited to see how our core expertise in clinical trial assessments is now transcending into the realm of precise and personalized patient care,” said Dr. Gideon Ho, Chief Executive Officer of HistoIndex.” With FibroSIGHT, we aim to empower clinicians with a more definitive and accurate assessment of liver fibrosis.”
MASH has long been a challenging and progressive liver disease, characterized by fat build-up and inflammation that, if left untreated, leads to fibrosis and, ultimately, cirrhosis. After decades of research and therapeutic development, the field reached a pivotal moment in 2024 with the approval of Rezdiffra® – the first drug for the treatment of MASH with moderate to advanced fibrosis1,2* . As treatment options expand, accurate fibrosis assessment becomes even more critical in guiding clinical decisions and optimizing patient outcomes. HistoIndex has been playing a key role in the development of MASH treatments and is now leading the way in this next phase of patient care with FibroSIGHT.
FibroSIGHT seamlessly integrates into routine clinical workflows, leveraging on HistoIndex’s proprietary stain-free imaging technology to enhance the sensitivity of fibrillar collagens detection — key in evaluating fibrosis severity in liver biopsy samples (see Figure 1). By eliminating variability associated with traditional staining techniques, FibroSIGHT delivers reliable and precise fibrosis assessment for MASH patients.
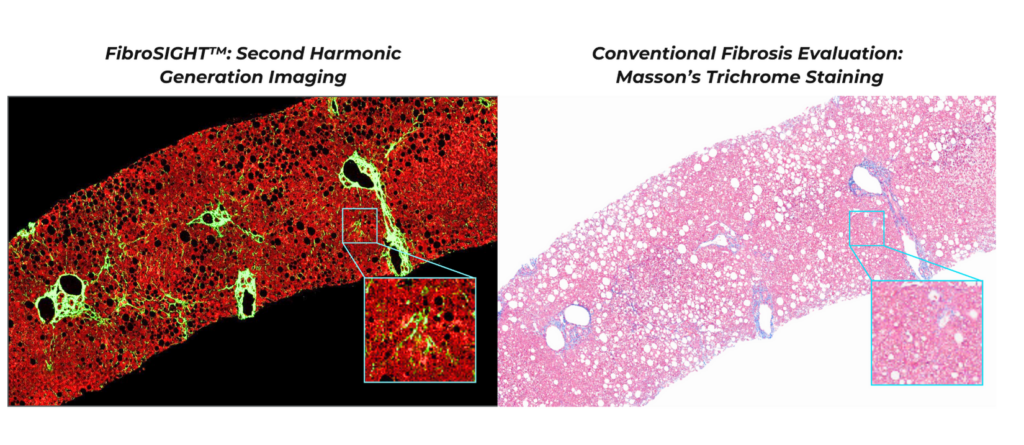
Figure 1: FibroSIGHT’s Second Harmonic Generation Versus Masson’s Trichrome Staining: FibroSIGHT enables detection of fine collagen fibers with exceptional clarity and an excellent signal-to-noise ratio.
Clinicians can now order FibroSIGHT for MASH patients, on whom liver biopsies were performed, whenever definitive and accurate assessments of fibrosis are needed2,3. This includes use at the time of diagnosis to determine treatment decisions, when non-invasive assessments of degree of fibrosis are either inconclusive or discordant. Additionally, FibroSIGHT can be ordered post treatment to evaluate patients’ response, especially in cases where there are no apparent improvement.
“By providing more accurate and objective evaluation of fibrosis, FibroSIGHT will enable more personalized treatment strategies and better evaluations of effectiveness of treatment leading to overall better care for patients,” commented Dr Naim Alkhouri, MD, Chief Medical Officer of Arizona Liver Health. “Where biopsy evaluation is needed for a MASH patient, I can see incorporating FibroSIGHT in the workup, and in doing so, benefiting the entire MASH clinical care community.”
FibroSIGHT testing is performed in HistoIndex’s CAP/CLIA accredited laboratory in Irvine, California. With the launch of FibroSIGHT, HistoIndex reaffirms its long-standing commitment in advancing fibrosis assessment and personalized treatment for MASH. By bridging research with clinical care, FibroSIGHT empowers informed treatment decisions, driving better patient care in the evolving landscape of liver disease management.
About MASH
Metabolic dysfunction-associated steatohepatitis (MASH) is a progressive form of Metabolic dysfunction-associated steatotic liver disease (MASLD) characterized by steatosis and inflammation, which can lead to fibrosis (scarring), cirrhosis, liver failure, and an increased risk of liver cancer. The presence of ballooned hepatocytes (enlarged and damaged liver cells) is a key feature distinguishing MASH from simple steatosis. Pathologist assessments of liver biopsy remain the gold standard for diagnosing and assessing the severity of MASH. Histological categorial scoring systems are often used as surrogate endpoints to evaluate drug efficacy in clinical trials. These endpoints are limited in capturing the complex and heterogeneous nature of the disease. As a result, there is a growing need for more accurate and reliable tools, such as AI-based digital pathology solutions, to improve the assessment of treatment response and disease severity in MASH.
About HistoIndex
Founded in 2010, HistoIndex pioneers in stain-free, fully automated imaging solutions for visualizing and quantifying fibrosis in biological tissues. By combining cutting-edge biophotonic technology with AI-based analysis, HistoIndex provides innovative tools to improve the assessment of fibrosis changes and drug efficacy. HistoIndex’s breakthrough digital pathology solutions are currently used in accelerating clinical research, expediting pharmaceutical drug development, and transforming medical standards
References:
1. U.S. Food and Drug Administration. (2024, Mar 14). https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease
2. Noureddin et al. (2024). Clin Gastroenterol Hepatol, 22(12), 2367-2377. (*consistent with stages F2 to F3 fibrosis, also referred to in the literature as “MASH with significant fibrosis,” “MASH and moderate fibrosis,” or “at-risk MASH.”)
3. EASL; EASD; EASO (2024). J Hepatol. 81(3):492-542.
