FibroSIGHT™:
A New Standard in
Clinical Care of
MASH Patients
Empowering clinicians with
accurate & consistent fibrosis assessment
What is FibroSIGHT™
Metabolic Dysfunction-Associated Steatohepatitis (MASH) is a liver condition involving fat build up and inflammation which left unchecked, leads to liver damage in the form of increasing levels of scarring or fibrosis, ultimately progressing to cirrhosis. The main risk factor for MASH is fibrosis: with each stage of fibrosis corresponding to a doubling of liver-related mortality1,2 but still, its evaluation is challenging due to limits of current methods3,4. After decades of research and therapeutic development, the field reached a true inflection point in 2024 with the approval of the first-ever drug for the treatment of MASH with moderate to advanced fibrosis5,6*. And now, accurate fibrosis assessment becomes even more crucial to determine the best treatment option for patients in routine clinical practice.
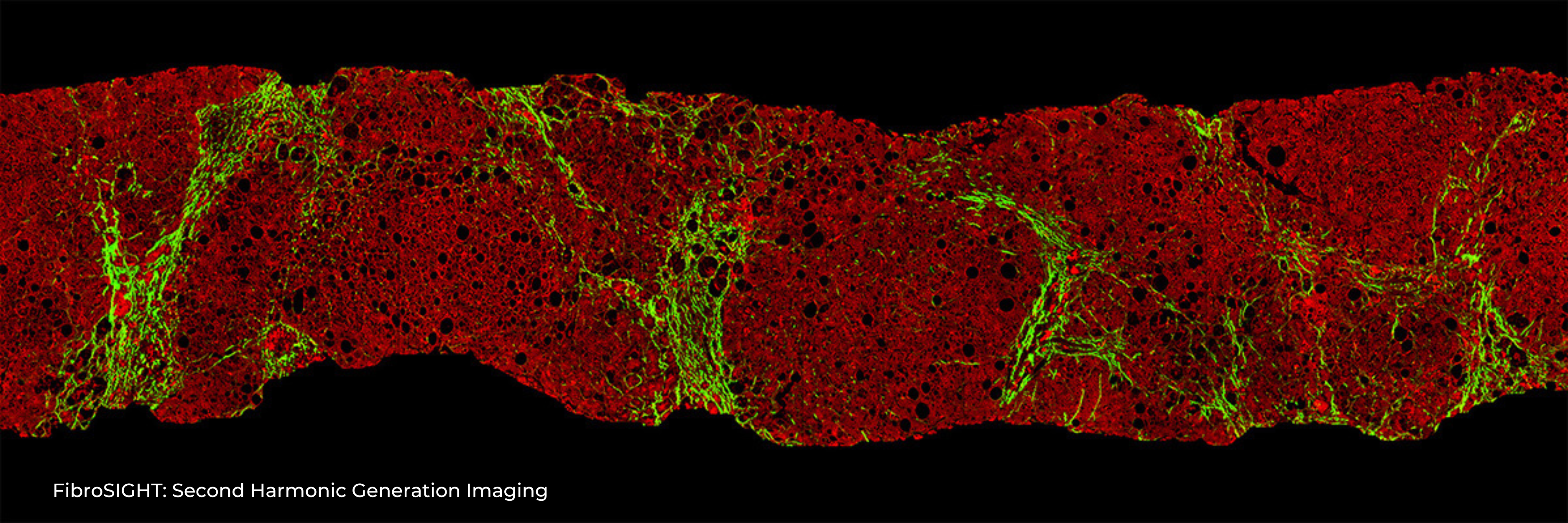
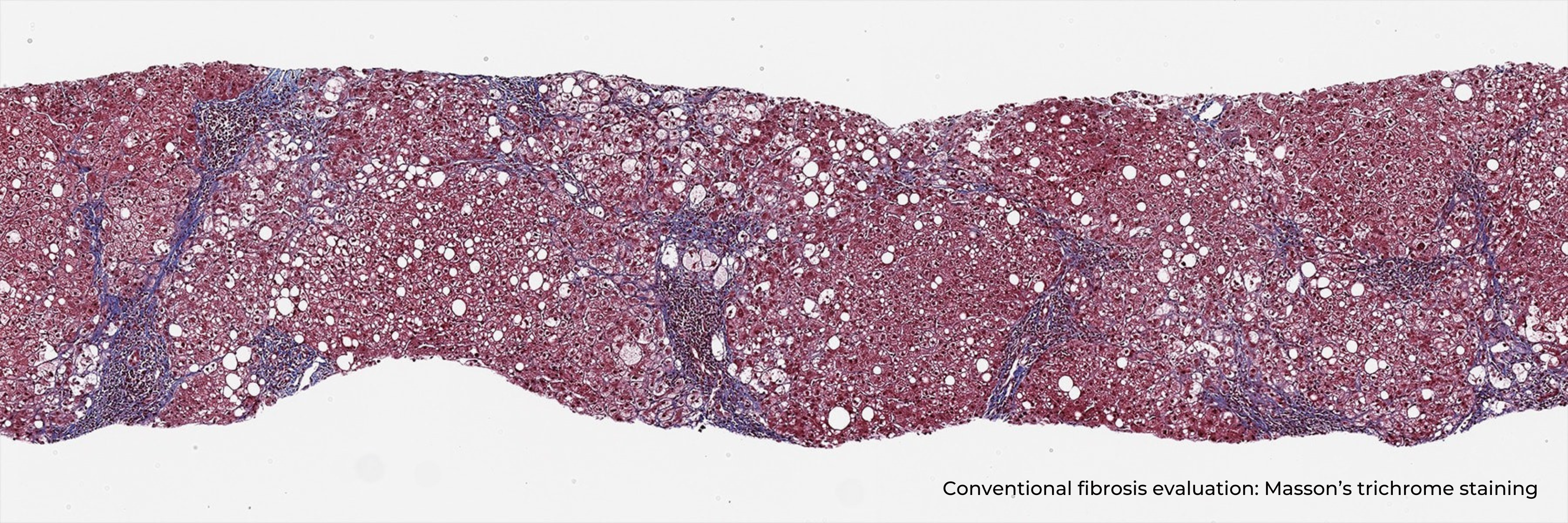
HistoIndex has introduced FibroSIGHT, a Laboratory-Developed Test (LDT), developed for reliable and consistent MASH fibrosis assessment. FibroSIGHT exploits a unique stain-free imaging methodology finely tuned to the sensitive detection of collagen fibers – an excess of collagen fibers is the underlying cause of fibrosis – in liver biopsy samples. FibroSIGHT overcomes the inconsistencies associated with traditional staining methods and can integrate seamlessly into existing histopathology workflows. FibroSIGHT’s imaging readouts have the ability to empower clinicians to more objectively evaluate a patient’s condition, help inform treatment plans at diagnosis and to assist in evaluating response to treatments – crucially addressing the central role of managing liver fibrosis in MASH patients.
Why Choose FibroSIGHT™
Accurate Assessment
FibroSIGHT enables sensitive detection and visualization of MASH liver fibrosis, eliminating the variability associated with traditional staining techniques. This greatly improves pathologists’ ability to evaluate fibrosis in liver biopsies. When using FibroSIGHT, pathologists show significantly higher agreement (with each other) on the degree of MASH fibrosis compared to standard assessments7,8.
Better Health Economics
Conventional pathology assessment of fibrosis is highly subjective, limiting resource-efficient patient care planning. By increasing accuracy, FibroSIGHT has the potential to increase the clinical yield from MASH liver biopsy evaluations, whenever biopsies are appropriate. With better matching of patients to the right treatment at the right time, healthcare costs and resource utilization can be potentially optimized.
Enhanced Disease &
Treatment Monitoring
FibroSIGHT provides an unparalleled detailed view of the progression and regression of liver fibrosis features, enabling clinicians to monitor disease status over time⁹.
This could support a more effective monitoring of treatment efficacy and response, ensuring timely adjustments to care plans.
Reliable &
Augmented Reporting
Every FibroSIGHT assessment, developed for standardized and dependable results, is performed by a board-certified pathologist and is grounded in the NASH-CRN staging system¹⁰. High-quality FibroSIGHT image readouts included in reports provide clinicians visual acuity on their patient’s fibrosis condition.
Innovative Technology Powering FibroSIGHT™
FibroSIGHT leverages HistoIndex’s advanced proprietary stain-free imaging and digital pathology platform developed on Second Harmonic Generation (SHG) and Two-Photon Excitation Fluorescence (TPEF). SHG imaging detects fibrillar collagen in biological tissues with consistency, high-resolution and excellent signal-to-noise ratio, while TPEF allows visualization of pertinent cell structures – all without the use of any exogenous dyes. This overcomes the limitations and variability of traditional staining methods.
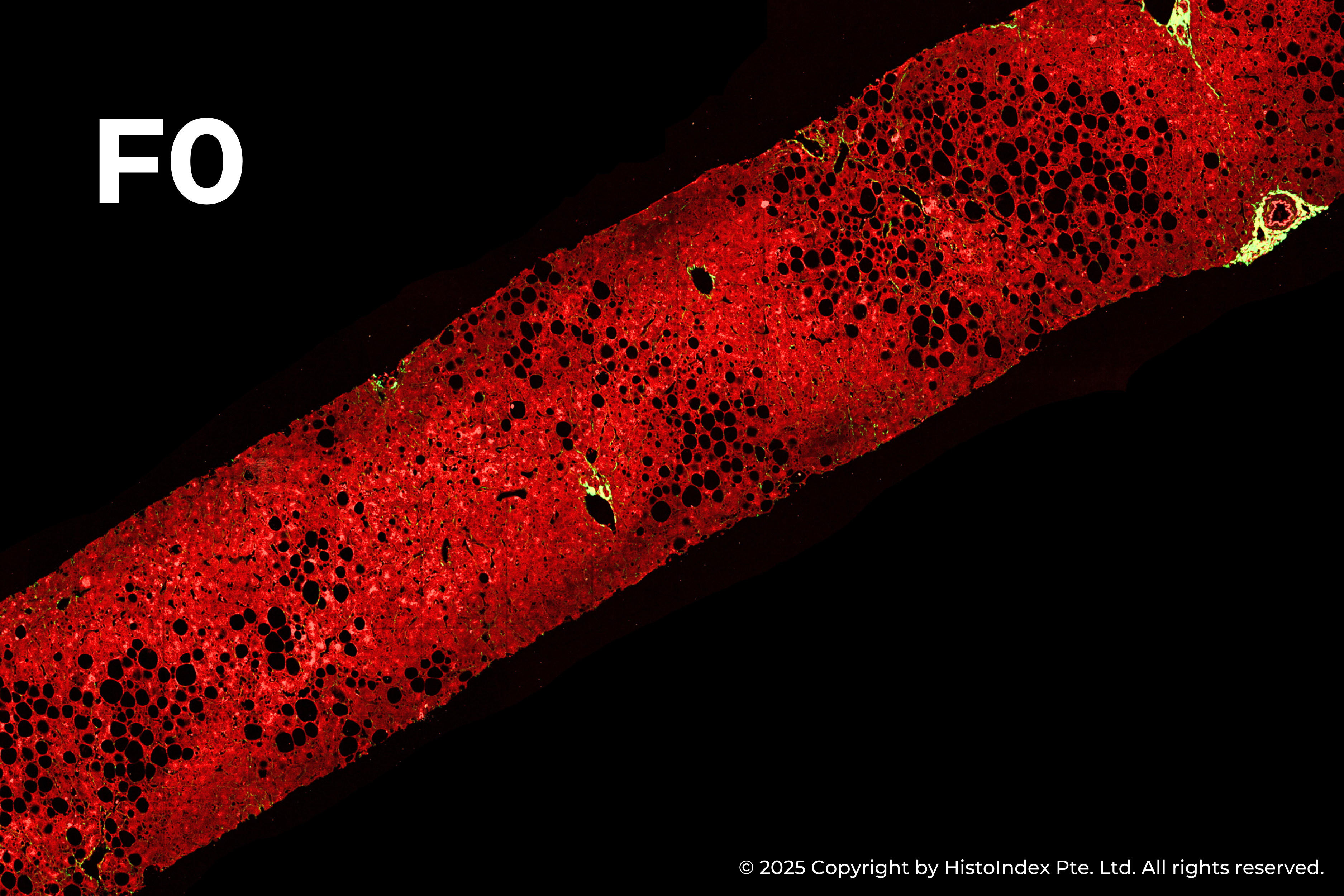
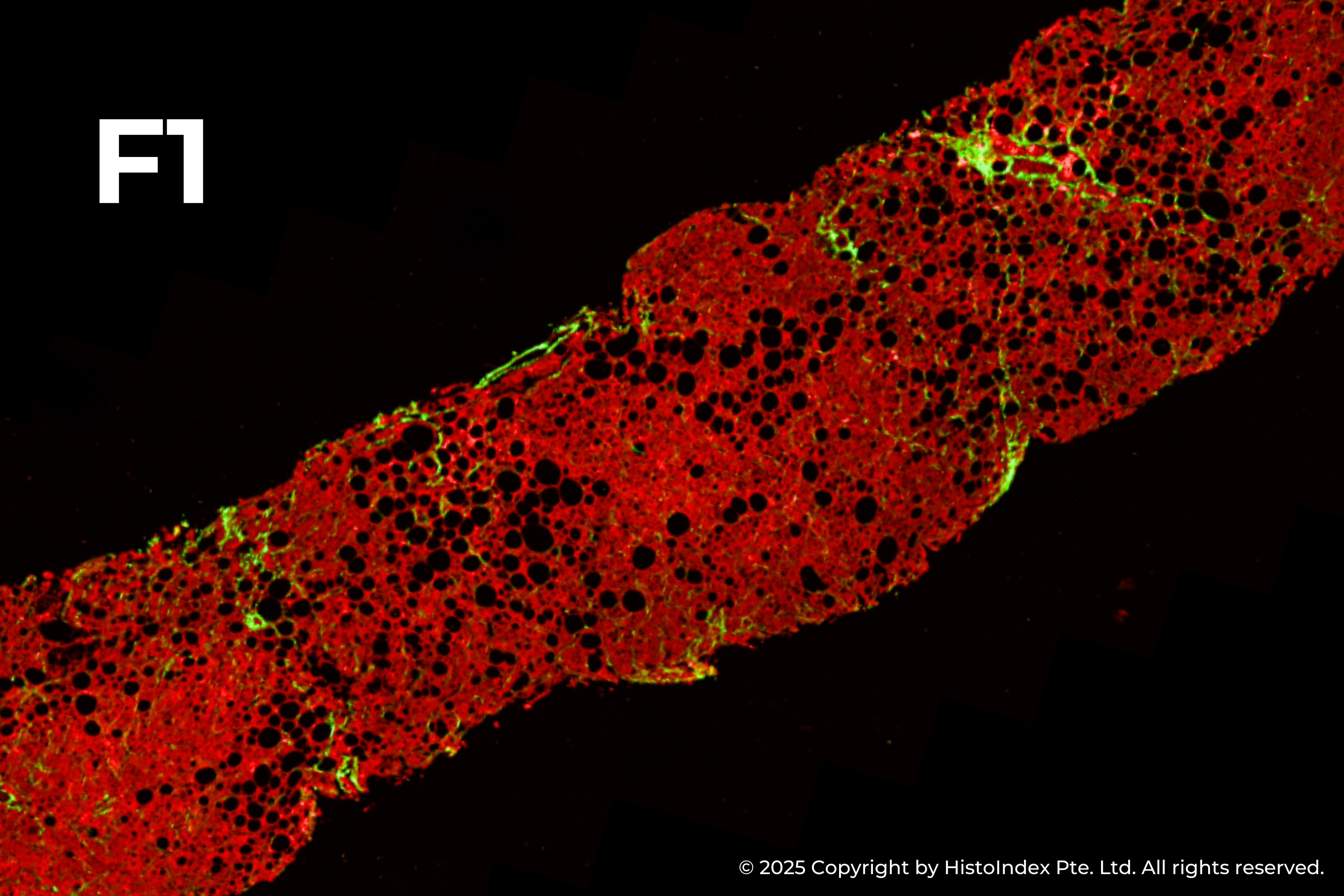
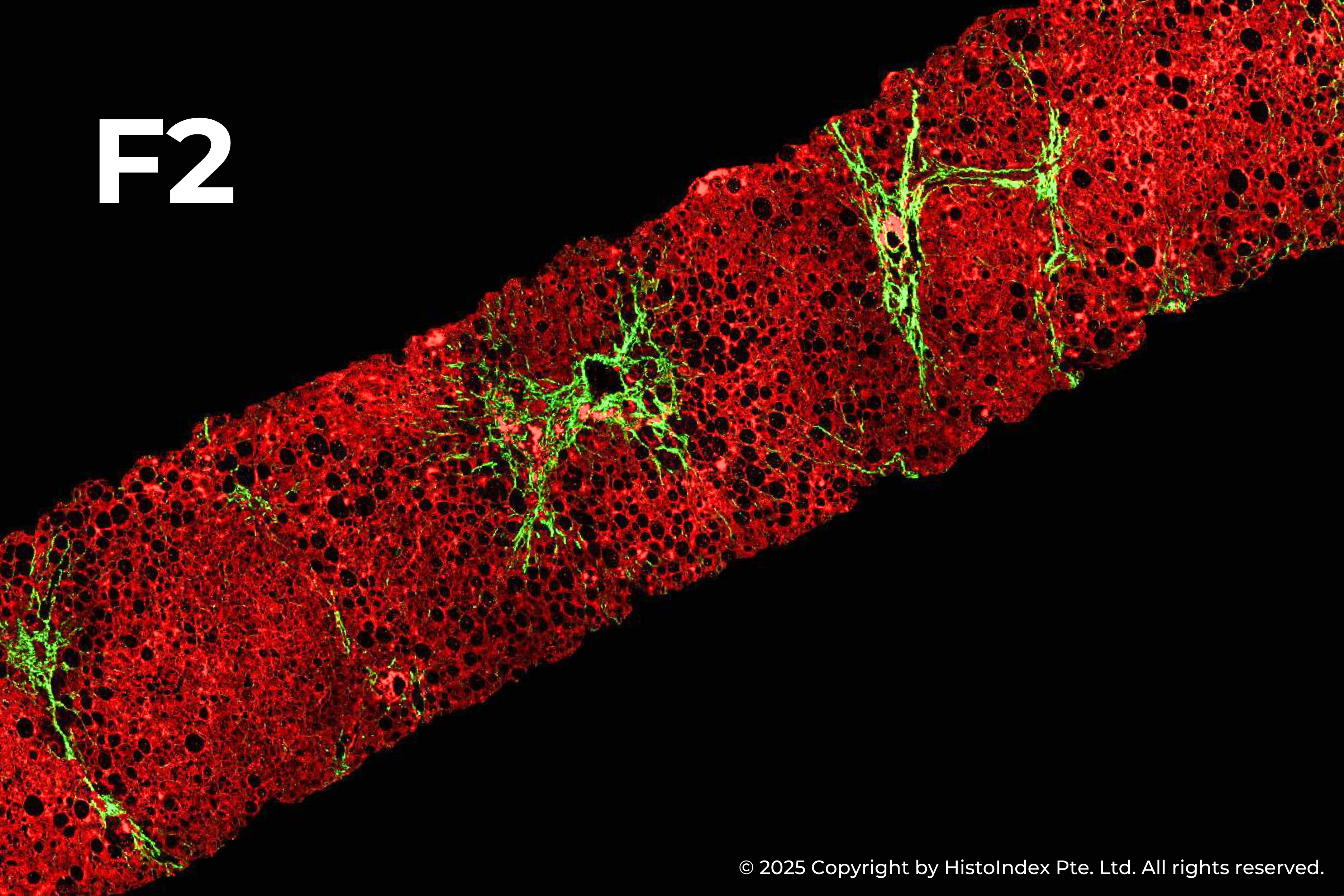
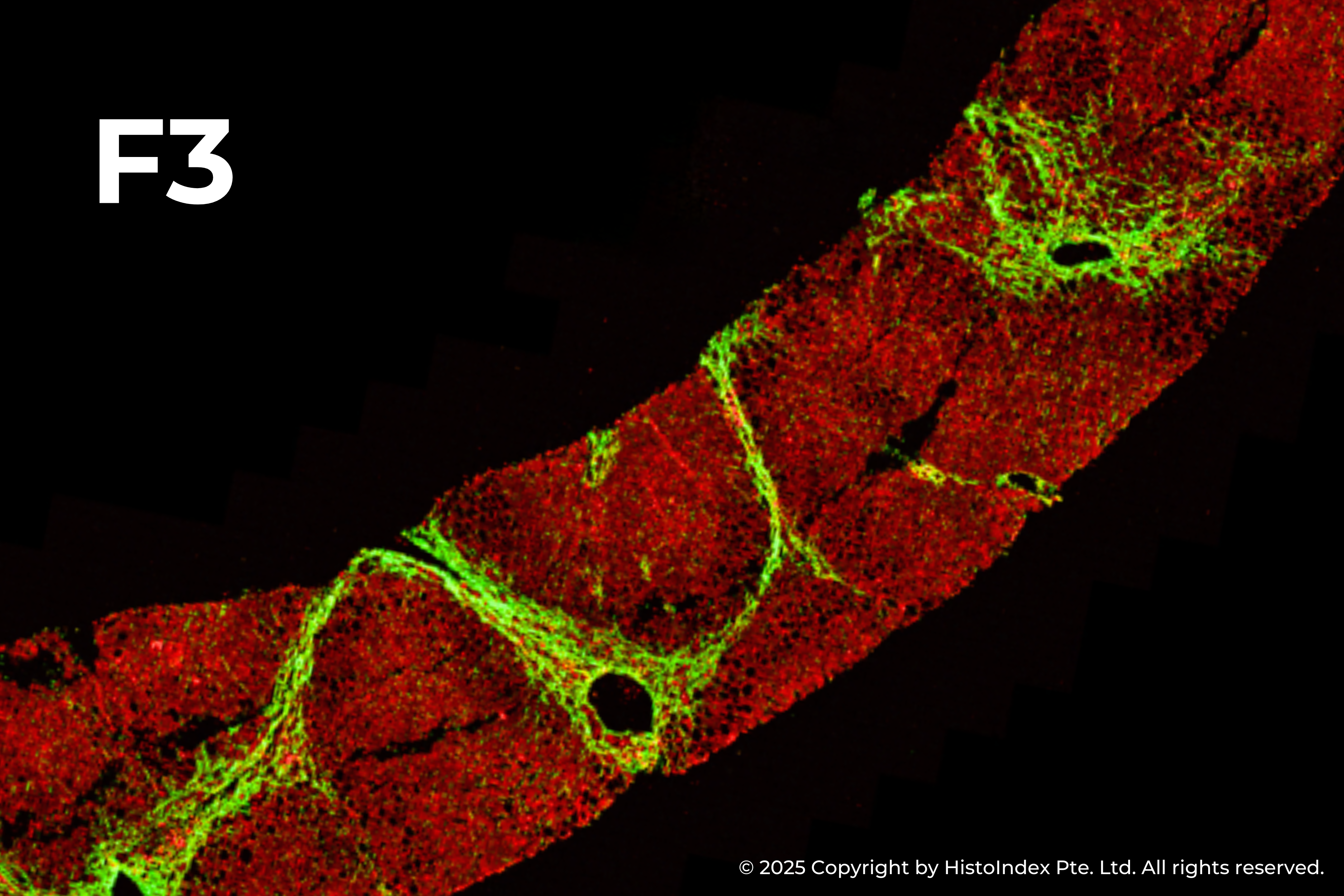
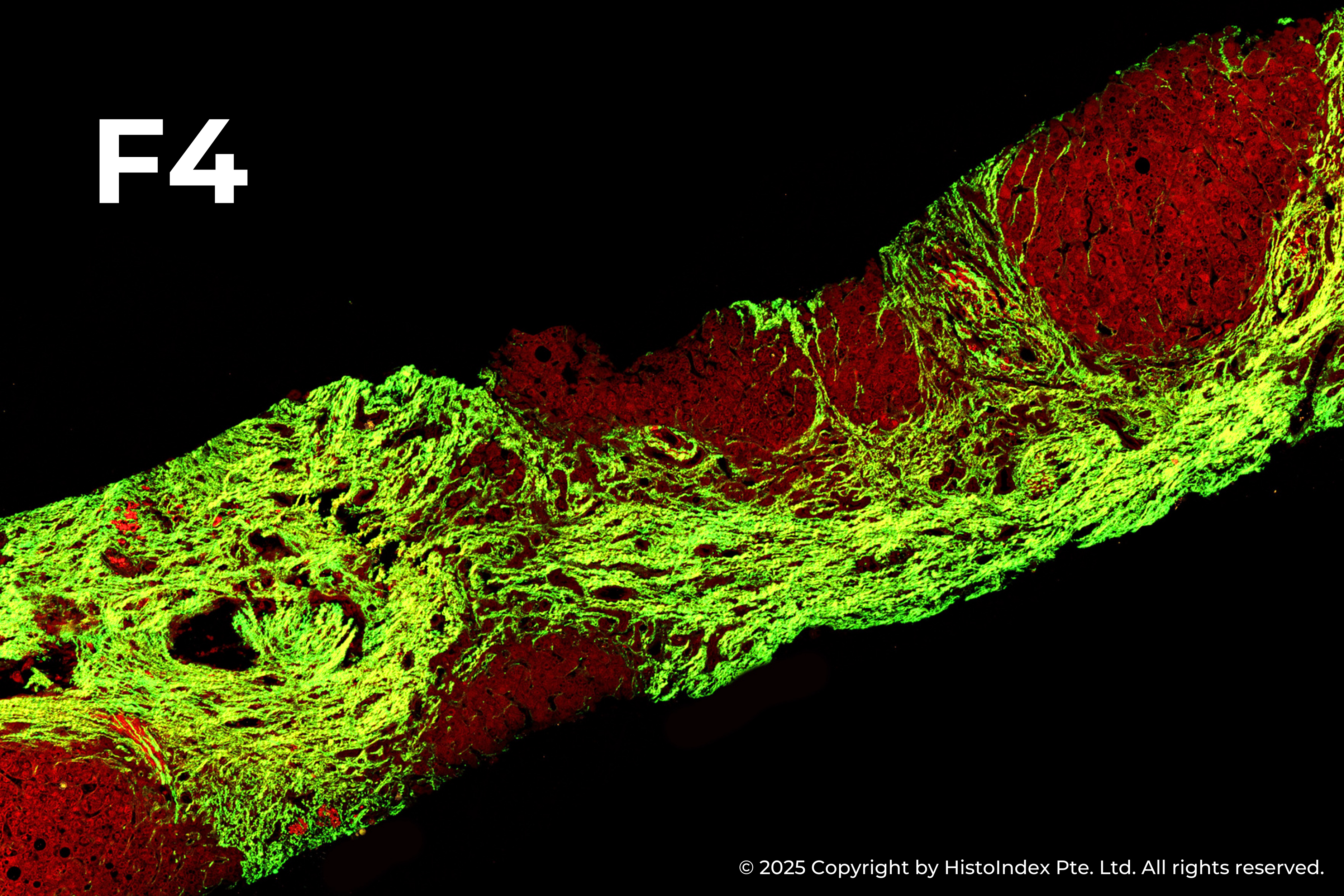
Liver biopsies of fibrosis stages 0 to 4, by NASH-CRN staging system
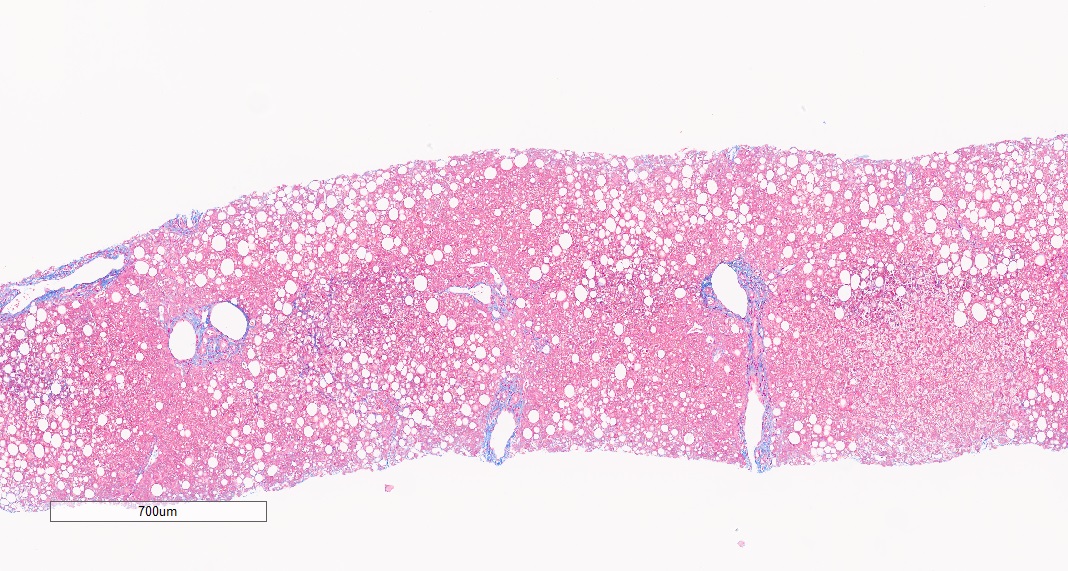
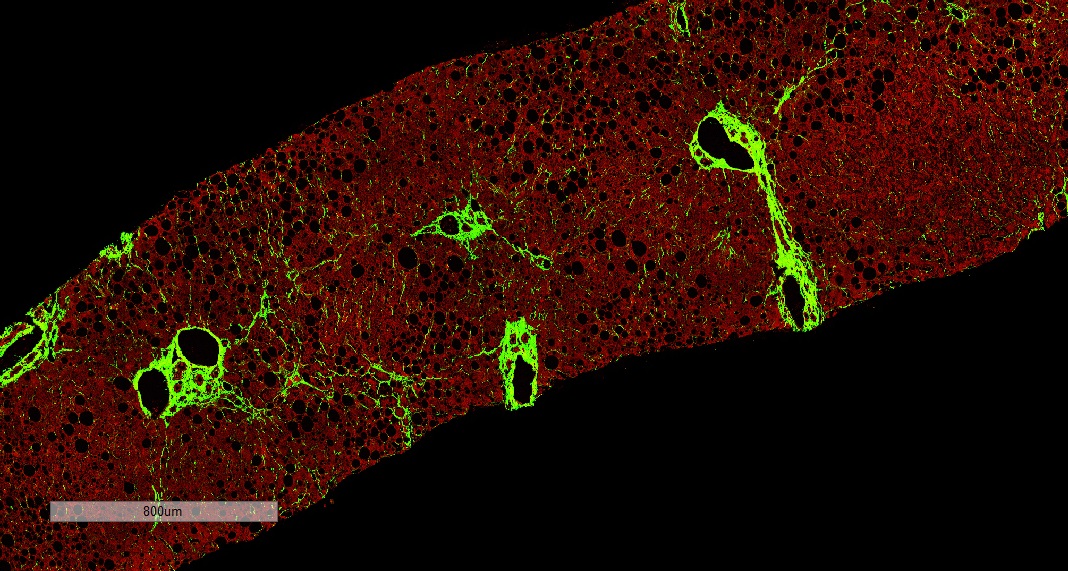
HistoIndex’s imaging technology has been at the forefront of fibrotic liver disease research for years. Precise quantitative measurements of fibrosis changes for drug efficacy determination in MASH clinical trials are made possible by the underlying consistency and quality of SHG stain-free imaging. With over a decade of expertise, we have scanned and analyzed more than 10,000 patient biopsy samples from MASH clinical trials in collaboration with leading pharma and biotech partners, often revealing insights of drug action not detectable by standard assessments9,11,12,13.
Refer to our publications page here.
Who May Benefit
from FibroSIGHT™
Clinicians order FibroSIGHT for MASH patients for whom liver biopsies are required for fibrosis assessment 6,14 :
1) At MASH diagnosis, to help inform treatment decisions
When non-invasive assessments of degree of fibrosis are inconclusive or discordant
2) After treatment, for patients not showing improvement
When non-invasive assessments show lack of improvement – to help understand treatment response
How FibroSIGHT™ Stands Out
Observe FibroSIGHT resolve equivocal fibrosis assessments in MASH patients:
CASE ONE
➤ VCTE indicated a high liver stiffness measurement of 13 kPa
➤ But, ELF score was low at 8.2
➤ Liver biopsy-based fibrosis assessment recommended
CASE TWO
➤ MRE reported borderline degree of liver stiffness of 2.9 kPa
➤ But, ELF score was high at 9.5
➤ Liver biopsy-based fibrosis assessment recommended
*These cases are hypothetical and meant for demonstration only.
How to Get Started
Step 2
Complete FibroSIGHT Requisition Form and send it to the pathology lab. Our team will work with the pathology lab to obtain liver biopsy sections
Step 3
Receive and review the FibroSIGHT report with your patient.
- Testing is performed by our partner CLIA /CAP accredited lab
- Available in the United States except specimens from New York state
- FibroSIGHT is validated on MASH liver biopsies only
- Full pathology services available – Contact Us
- Terms & Conditions apply
Quick Answers
As sample quality can vary, we recommend sending 3-5 unstained FFPE slides for FibroSIGHT testing.
A report will be sent to you within 7 working days from sample receipt in the laboratory.
A board certified pathologist will issue the FibroSIGHT report.
Samples can be shipped from any US state with the exception of New York. Our courier services will take care of the sample shipment.
We will coordinate with the patient’s insurance provider to provide coverage for FibroSIGHT testing. However, the patient may be responsible for out-of-pocket expenses including co-payments, co-insurance or deductibles depending on the patient's insurance plan. We also provide cash pay for FibroSIGHT testing.
FibroSIGHT Testing is performed in our CAP/CLIA accredited lab in Irvine, California.
Yes! We offer full liver pathology services so we can provide more comprehensive care to patients. Contact us to find out more.
No, FibroSIGHT testing is done independently of H&E and MT staining. If desired, additional pathology services are available from our laboratory upon request.
Contact Us
Read Website Terms of Use Here
References
1. Dulai et al. (2017). Hepatology, 65(5), 1557-1565.
2. Sanyal et al. (2021). N Engl J Med, 385(17), 1559-1569.
3. Davison et al. (2020). J Hepatol, 73(6), 1322-1332.
4. Anstee et al. (2022). J Hepatol, 76(6), 1362-1378.
5. U.S. Food and Drug Administration. (2024, Mar 14). https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease
6. Noureddin et al. (2024). Clin Gastroenterol Hepatol, 22(12), 2367-2377. (*consistent with stages F2 to F3 fibrosis, also referred to in the literature as “MASH with significant fibrosis,” “MASH and moderate fibrosis,” or “at-risk MASH.”)
7. Abdurrachim et al. (2024). J Hepatol. https://doi.org/10.1016/j.jhep.2024.11.032
8. Soon et al. (2023). Clin Gastroenterol Hepatol, 21(7), 1940-1942.
9. Naoumov et al. (2022). J Hepatol, 77(5), 1399-1409.
10. Kleiner et al. (2005). Hepatology, 41(6), 1313-1321.
11. Sanyal et al. (2023). Nat Med, 29(2), 392-400.
12. Harrison et al (2023). J Hepatol, 78, S112.
13. Noureddin et al. (2024). [Poster presentation]. The Liver Meeting 2024 (AASLD).
14. EASL; EASD; EASO (2024). J Hepatol. 81(3):492-542.
